The discovery of protons is credited to Ernest Rutherford. During chemical reactions atoms rearrange to make different substances This atomic model has changed over time.

Particle Theory Changes Of State
Occurring in the nucleus of the atom when it emits or absorbs rays or particles when the nucleus splits or joins with another State the kind of change in matter in each example below a.
. 5 ways to recognize chemical changes 1. Hence an atom is electrically neutral. How did JJ Thomson change the atomic model.
We use multiple models of atoms to help explain chemical processes and describe their. A As the masses of protons and neutrons are greater than electrons most of the mass of an atom is concentrated in the nucleus. Alpha particles are positively charged so deflections arose when they encountered another positive charge like charges repel each other.
Production of heat and or light 2. In this way we can describe the atom clusters. Here is what Rutherford deduced.
The particles were about 2000 times lighter than the hydrogen ions. Atoms combine to form molecules which then interact to form solids gases or liquids. If the positive alpha particles mostly passed through the foil but some bounced back.
The atoms of a substance are rearranged to form new substances. This model was known as the plum pudding model. Ocean waves and sand erode a sea cave in a solid rock cliff b.
Particles stay the same unless there is a chemical change whether the matter is solid liquid or gas. Atoms are incredibly small and cannot be seen with even the most powerful light microscope. Based on his observations here is what thomson proposed and why.
Only their arrangement energy and movement changes. The size of these ultrasmall particles is usually millionths of a millimeter or even smaller. Thomsons experiments with cathode ray tubes helped him to discover the electron which Dalton did not know about.
This theory was then disproved by Ernest Rutherford and the gold foil experiment in 1911 where Rutherford shot alpha particles at gold foil and noticed that some went through and some bounced back implying the existence of a positive nucleus. Production of a gas fizzing or bubbling 3. Occurs when a new substance with a composition different from that of the original substance forms.
AND if they already knew that the electron was. B The nucleus has an overall positive charge due to the positively-charged protons in it. Rutherfords experiment prompted a change in the atomic model.
The model of the atom has dramatically changed over many many yearsWe learned atoms make up different substances and are the smallest particles of matter which have subatomic particles that are very small portions of matter. The number of protons in an atom is equal to the number of electrons in it. Sometimes the results of.
The clusters are studied in various fields of nanoscience and they are used for example in chemistry to several purposes. We change the properties of particles atom by atom so that their performance. When substances change state there is no change in mass so if 100 g of ice is melted 100g of water are formed this will boil to form 100g of steam this is called conservation of mass.
Protons are positively charged subatomic particles. Chemical changes involve chemical reactions and include. Because most of the fast-moving α particles passed through the gold atoms undeflected they must have traveled through essentially empty space inside the atom.
C An atom consists of an equal number of electrons and protons. JJThompson named the particle as corpuscle but the name was later changed to electron. It is equal in mass to a proton or it weighs 1 amu.
Dalton thought that atoms were indivisible particles and Thomsons discovery of the electron proved the existence of subatomic particles. Click to see full answer. How the Model of an atoms has changed.
Formation of a precipitate solid 4. Methane burning in air forming carbon dioxide and water magnesium reacting with. An atom is the smallest unit of matter that retains all of the chemical properties of an element.
2 Show answers Another question on Chemistry. The particles are attracted by positive charges and repelled by negative charges so they must be negatively charged like charges repel and unlike charges attract. The mass of a proton is 1676 10 -24 grams.
At first scientist only thought there were electrons which are negatively charged. Even neutrons can can change to a proton. Protons can be produced via the removal of an electron from a hydrogen atom.
The only really stable particles in the absence of anti-matter are the proton and. When a block of ice is taken out of a freezer the sudden change of temperature reacts on the particles making them decrease in size Scientific view. Since the electrons originated from within the electrodes they led Thompson to conclude that atoms were divisible and that electrons corpuscles were the building blocks of the atom.
A subatomic particle forming part of the nucleus of an atom. They are less massive than atoms and indistinguishable regardless of the source material so they must be. Some decay into other particles in an incredibly short period of time.
Describe how changing the particles changed the atom. Daltons actual model of the atom was uncharacterized other than to suggest that atoms were the building blocks of. It has no charge.
The discovery of subatomic particles proved that atoms were not indivisible. Scientists used the model to make predictions.

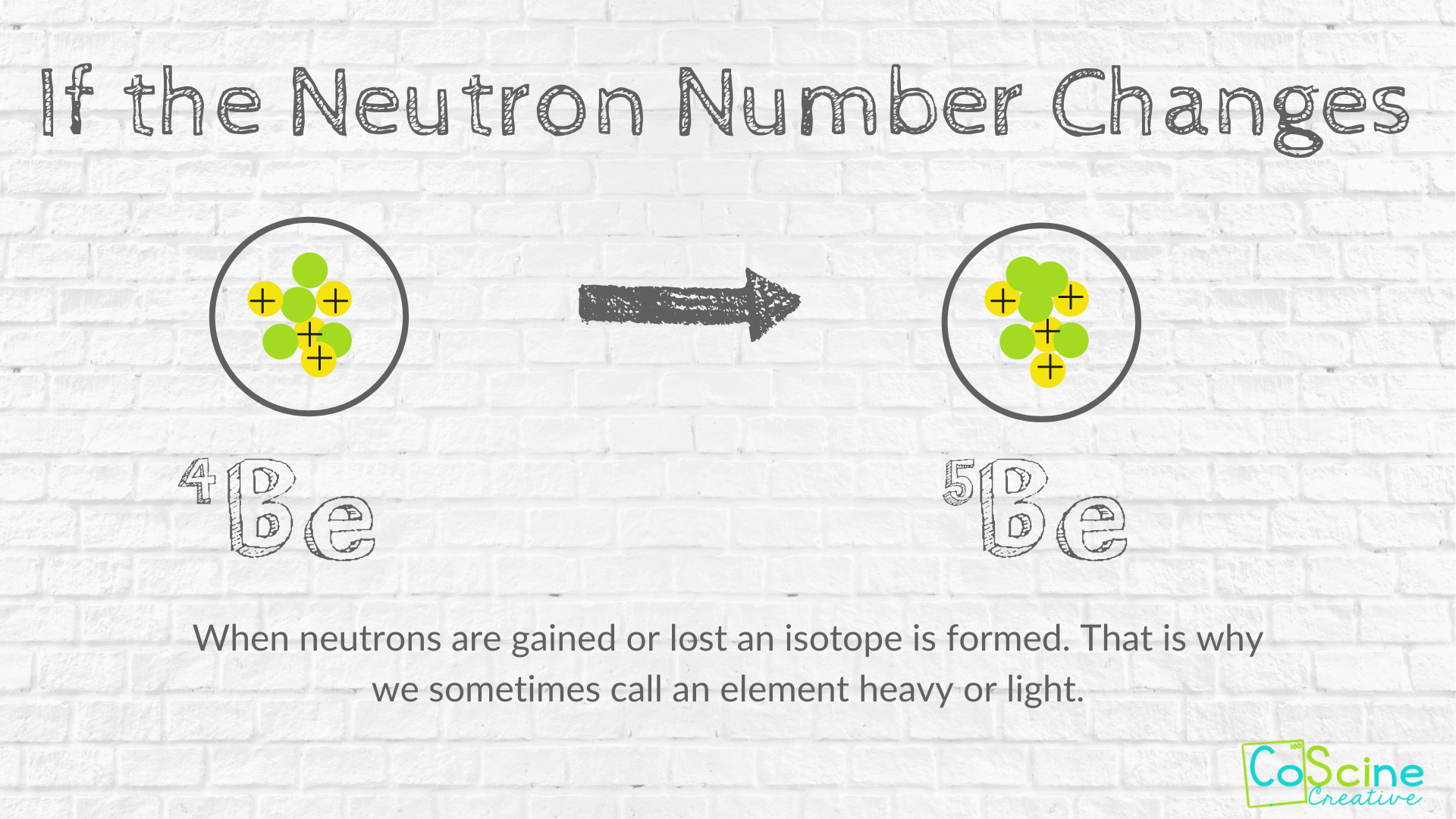
How Does Changing Numbers Of Subatomic Particles Effect The Atom Coscine Creative
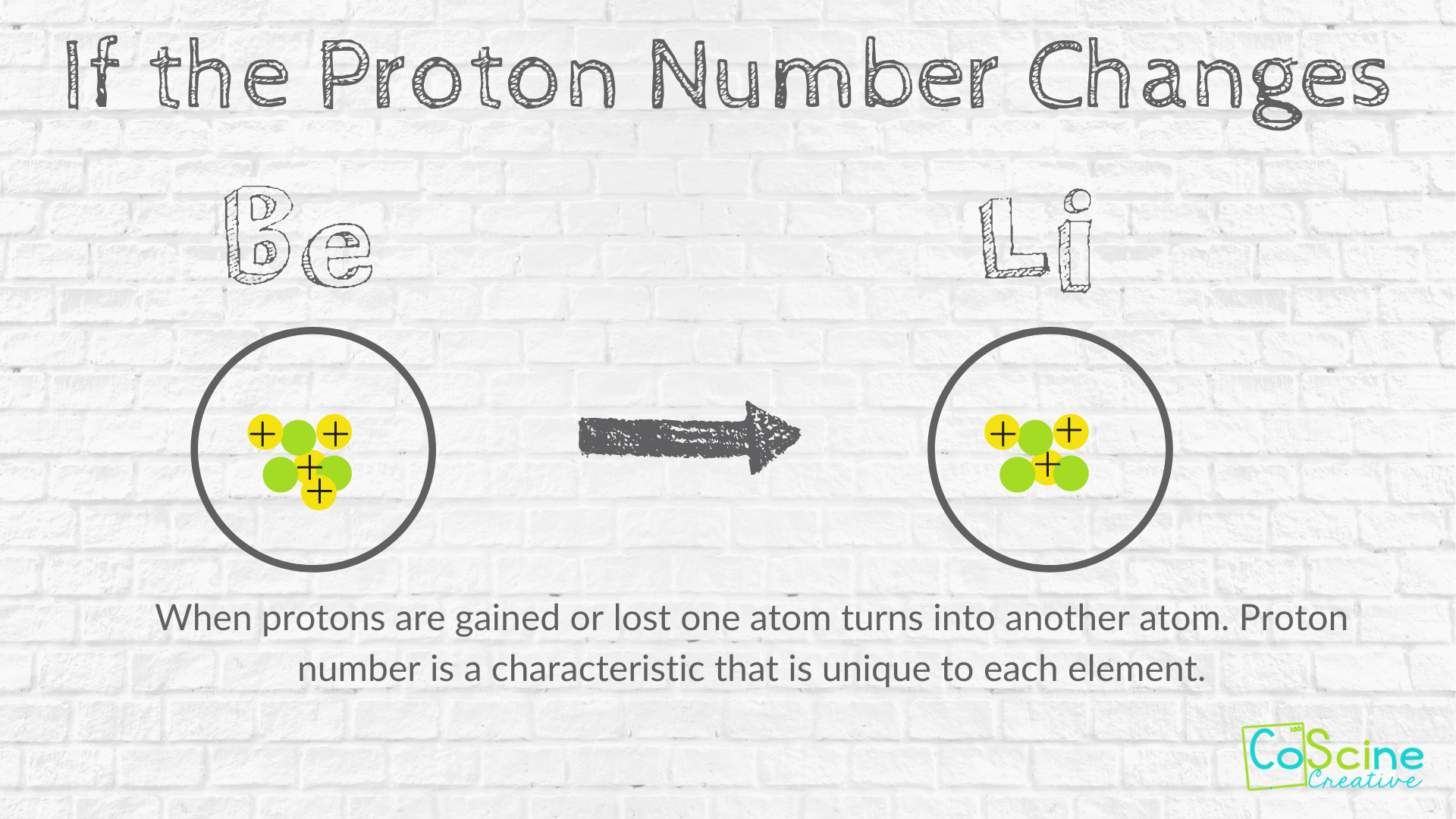
How Does Changing Numbers Of Subatomic Particles Effect The Atom Coscine Creative

How Does Changing Numbers Of Subatomic Particles Effect The Atom Coscine Creative

What Are The 5 Postulates Of Dalton S Atomic Theory Atomic Theory Chemistry Lessons Study Chemistry
0 Comments